The Age Of The Earth
These are the notes from the last session of a six-session course I taught in November 2012 in the West region of the Chicago Church of Christ. This session is inspired by a class taught by Dr. Willis Hames during an International Apologetics Conference in 2007 in Elmhurst, Illinois, hosted by the Chicago Church. Dr. Hames is a professor of geology at Auburn University and a Christian since 1990.
The primary text is “The Age of the Earth”, an authoritative introduction for the lay-person, written by G. Brent Dalrymple in 1990.
Pre-class homework: Excel Sheet Isochron Model
How can we expect unbelievers to trust our statements about spiritual things if we make outlandish statements about worldly things? –Augustine of Hippo, 354-430 AD
Why should you care about the age of the earth? [class response]
Guard against dogmatism in our faith. This issue spurs us to wrestle with the Bible and our faith in perhaps a new way, which keeps us from making foolish statements about earthly things, and so protects our credibility in the eyes of men and makes us more effective witnesses of our Lord to our own generation.
Help others’ faith, especially our children’s. It equips us to defuse what I believe is a time bomb in Western Christian thought: a huge disconnect in many peoples’ minds between the Bible and science. It disarms one more tool of Satan that he would use to discourage people from seeking the truth. We are then in a position to hold beliefs that command respect, instead of being dismissed as out of touch and unable to think clearly. In that case, we would be out of step with Stephen in Acts 6:9…
They could not stand up against his wisdom or the Spirit by whom he spoke.
And Jesus in Mt 22:46:
No one could say a word in reply, and from that day on no one dared to ask him any more questions.
I am not saying we should become champions at arguing and debating. However, when push came to logical shove, Stephen and Jesus went toe-to-toe with their opponents, and they prevailed based not mainly on their personalities, but by the wisdom and knowledge they displayed–the force and soundness of what they were saying!
Know God better. We can know a lot about a person by looking at his work. [Read Jeremiah 33:17-26]. Through the Spirit, Jeremiah appeals to the “fixed laws of heaven and earth” as evidence that God is faithful to his promises. If we view God as “magic” or capricious in his creation, often suspending or overriding his own fixed laws in order to accomplish his purposes, then we will hold a very different understanding of him than if we see him abiding by his own established order except in very rare cases. Is God really so short-sighted that he would “paint himself into a corner” and be forced to break his own laws in order to do his best work? If he doesn’t take his own word seriously, then why would we? Jeremiah for one did not see God this way.
Practice holding a unified view of truth. Studying the age of the earth is a wonderful antidote for the cognitive dissonance of post-modern thinking, since (a) a vast quantity of scientific data is available on the topic, and (b) the Scriptures leave ample room for a view that accommodates both Scriptural and scientific knowledge.
Goals:
Motivate us to mature in our understanding of science and faith. We need to correctly handle the word of truth (2 tim 2:15) in order to win as many as possible.
Encourage us to not be suspicious or paranoid of science or scientists , nor afraid to embrace what valid scientific knowledge provides us in seeing the wonder of God’s creation. “The heavens declare the glory of God…night after night they display knowledge”. Ps. 19
Exhort us to integrity in our approach to science and faith. We must guard against on the one hand referring to science to further our own agenda, while on the other hand discounting scientific evidence that challenges our preconceptions.
Today we will be looking at isochrons - one example of the modern techniques available for dating rocks to a remarkable precision.
Knowledge of the age of the earth has come a long way!
Scientific research has shed considerable darkness on the subject of the age of the earth, and if they continue at their present pace, we’ll soon know nothing about it.
–Mark Twain (1835-1910)
Much has changed since that time. From a tremendous body of research and discovery, we now know that a countless number of rocks come with very precise clocks. Until the 1960’s, we couldn’t read these clocks with any degree of confidence.
With today’s material, I hope to lay a solid foundation for the position that the scientific case for an old earth is extremely strong–approximately as strong as the case for a spherical earth. As I discussed in the previous session, the Bible does not teach against an old earth. On the contrary, we observe that…
- Genesis 1 and Genesis 2:4 use the same Hebrew word for “day” to mean different lengths of time, depending on context.
- Those chapters describe creative processes that take long periods of time, and that rely on the abilities of non-miraculous entities (the land and man, for instance) to partner with God in significant portions of creation.
- The seventh day–the day of rest–is still open, and so is the invitation to enter it (Hebrews 4); no boundary has yet been defined.
Why do so few mainstream scientists bother to engage creation science? Douglas Jacoby, a prolific Christian teacher and author in the International Churches of Christ, concludes that they simply don’t see any benefit to entering the discussion:
Many scientists are Bible believers, and many are not, but extremely few adhere to the conclusions and antics of the “scientific creationists”… The great antiquity of the earth was well established– even among churchmen –nearly a century before Darwin. All dating methods [past and present] yield great ages for the earth. Arguments creationists use for the “refutation” of dating techniques which consistently yield a great age for the earth, the solar system and the entire universe, are ludicrous to the point of embarrassment.
–Douglas Jacoby, “Genesis, Science, & History”, p. 111
G. Brent Dalrymple, one of the foremost authorities on the age of the earth, agrees. By his own account, he got involved only because he was asked to testify in a court case. Up to that point, he considered the claims of creation scientists too bizarre to even engage. [AotE, pp. vii-viii].
By the way, one more difference between Galileo’s earth-goes-around-the-sun claim and the present-day old-earth claim that we didn’t mention: We have at hand a massive quantity of data , compared to Galileo’s tiny body of evidence.
Let’s learn about radiometric dating…
By the end of class, you will know what this sentence means, and why it is important:
The chemical processes of crystallization do not fractionate isotopes of the same element.
Who has read the TalkOrigins article on isochrons?
Agenda for today
- Difference between a rock and a mineral
- Difference between an element and an isotope
- Difference between chemical and nuclear reactions
- Radioactivity:
- Definition of half-life
- Quick comments on decay rates
- What is meant by “old”?
- What happens to a rock when it cools
- “direct” (aka “generic”, or “straight”) method of dating
- The “isochron” method of dating–solves two fundamental problems of direct dating:
- The initial-daughter problem
- Cross-checking whether a rock has been a closed system
Difference between a Rock and a Mineral
A rock is an aggregate of minerals
A mineral has a crystalline structure and a characteristic chemical composition–a formula.
Minerals form when molten rock cools and crystalizes.
Difference between an element and an isotope
Atomic number vs. atomic mass: Different isotopes have the same same chemical properties (we will see in a moment why this is very important for radioactive dating techniques), because they are the same " element"–that is, same number of protons i.e., atomic number. However, isotopes of a given element differ in atomic mass , that is, roughly the sum of protons and neutrons. Isotopes of an element behave the same chemically, but can be distinguished by radiometric techniques.
84 elements are found in nature, consisting of 339 isotopes. 70 of those isotopes are radioactive. 18 long-lived isotopes have long half-lives and have survived since the beginning of the Solar System. The other 52 have short half-lives, but are being continuously regenerated by ongoing processes. 1,650 or so others are “missing”–more on that later.
Over time the radioactive isotopes change at very constant rates into other isotopes and/or elements. How constant? What about strong electric fields, temperature, pressure, gravitation, other radioactive processes, etc.
…no changes [in the decay rate] have ever been detected for any of the isotopes used in radiometric dating, and none of significance are theoretically expected.
–AotE, p. 89
How old is “old”? What do we mean when we talk about a rock’s “age”?
Nearly every atom in our bodies has gone through several stars and millions of organisms since the beginning of creation!
If this is the case, then when is a rock “born”? A rock is born when molten rock cools and becomes solid.
The direct approach to dating a rock: Just measure the amount of an element and its decay product (aka “daughter”) directly.
Drawbacks of direct method:
- Impossible to know how much daughter initially present (in some cases).
- Difficult to cross-check the results.
The basic radiometric age equation:
t = 1/Alpha loge((Dt - D0)/Pt + 1)
…where Alpha is the decay constant for the given isotope, and t is the age of the mineral in years.
How do we know how much D0 (initial daughter) is present?
We CAN do this with some elements, if we know its initial concentration is zero (For instance, Argon: in general, all the argon escapes from molten rock before it cools and becomes solid. Therefore, any Argon-40 present in solid rock must have come from the radioactive decay of Potassium-40.)
(Another problem: What if the rock is not a closed system? That is, what if D or P was added or subtracted some time in the past, after the rock formed? We’ll deal with that in a moment. But first…)
Difference between chemical and nuclear reaction
What defines the chemical properties of an element? Its protons and electrons. For nearly all practical purposes, the neutrons are chemically neutral.
Recall our sentence with all the big words…
“The chemical processes of crystallization do not fractionate isotopes of the same element.”
(Or, if you prefer: “Mineral-forming processes can’t distinguish between nuclides with the same number of protons.” )
Although difficult to turn into a catchy phrase, this property removes the need to know the daughter element–which is the key to successful radiometric dating of most rocks. Let’s break it down…
When a rock is liquid, everything mixes homogeneously, that is, evenly throughout the substance. And when it cools and forms minerals–that is, when it crystallizes –radioactive isotopes of the element behave chemically exactly like non-radioactive isotopes of the same element. In other words, the isotopes of a given element don’t separate from each other, or fractionate , as a result of their isotopic properties (of course, a given element will fractionate into minerals during cooling based on its chemical properties; one type of mineral will have a higher percentage of carbon than another, for instance).
This is fantastic! Why? Because if we take one more measurement, which is the amount of NON-radioactive daughter that is there now, at the present time (Dt), we no longer need to know D0 (“D-zero”), the daughter element’s initial concentration.
Why don’t we need to know D-zero? This brings us to the isochron diagram,
…a device of magnificent power and simplicity. –AotE, p. 102
Why are isochrons so cool?
- They solve the “initial daughter” problem - no need to know how much D-zero we started with
- They come with built-in cross-checks
- The cross-checks built into an isochron show clearly whether the rock is “undisturbed”, a closed system. The “fit” of the data to a straight line can be measured statistically to establish a probability that the measurement is good. In other words, straight line = good, crooked line = not good.
- The Y-intercept of the isochron, no matter what the slope, can easily be used to determine the age of the rock.
Let’s see how an isochron works…
[Explain the isochron diagram, from p. 102]
http://www.talkorigins.org/faqs/isochron-dating.html – A succinct summary of isochron dating techniques.
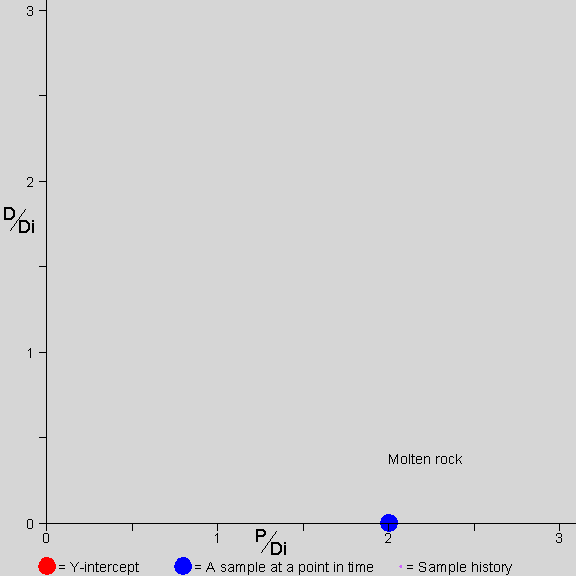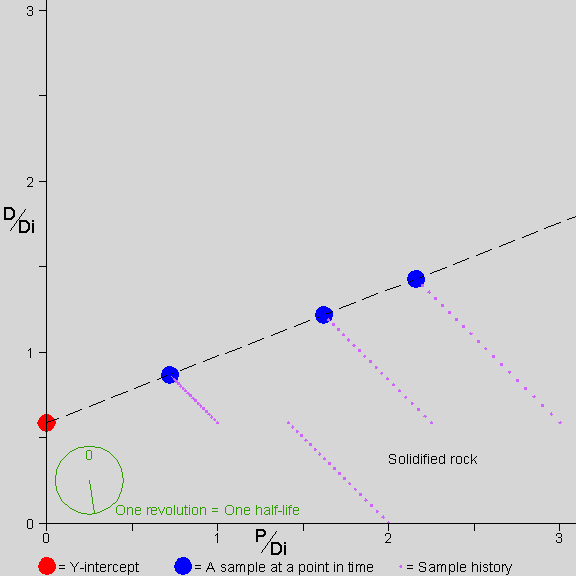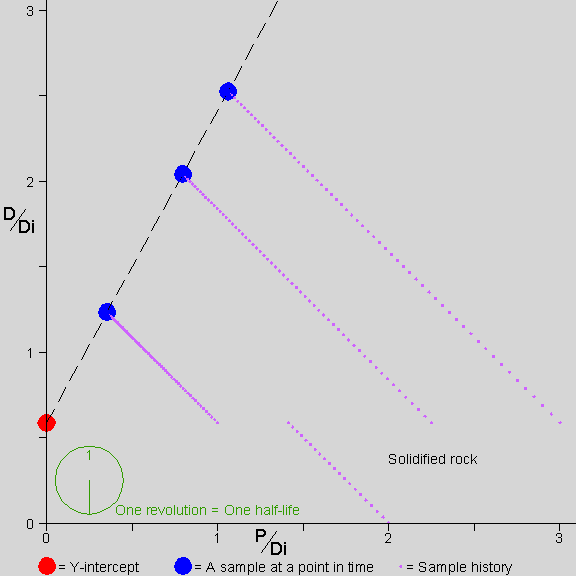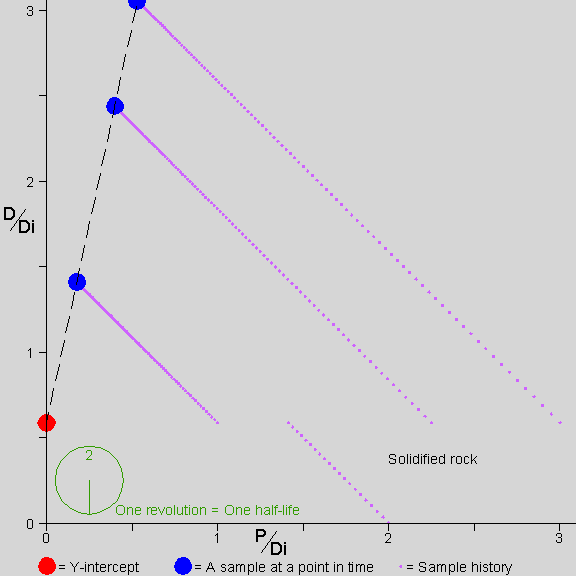
This isochron animation is from a page by Jon Fleming on www.talkorigins.com. Click here for an explanation of what’s going on as a rock forms and ages.
[Work through the exercise of normalizing Daughter-vs-Parent graph by the non-isotope of Daughter. page 104 in Dalrymple]. In a nutshell, even though the amounts of daughter element differ w.r.t. each other in a rock’s complement of minerals, the ratios of daughter element in those component minerals remain constant with respect to each other–and these identical ratios are highlighted by the straight line of an isochron.
Download the Excel sheet:
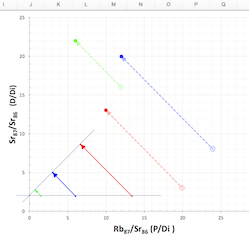
Since an isochron measures the LAST time that a rock became solid, the age given by a valid isochron is the youngest possible age of the rock.
The case of the missing nuclides
A nuclide is a term for referring to an atom based on the number of protons and neutrons in its nucleus.
Only nuclides with half-lives longer than 82 million years are found in nature. The exceptions are those produced by natural processes, i.e., U236, I129, Mn53, Np237, and Be10.
–AotE p. 376
Three possibilities can explain this absence:
- It’s just random chance that they never formed,
- The element-forming processes couldn’t produce the “missing” nuclides, or
- Enough time has passed that the nuclides have simply decayed away.
Number (1) is easily disproved. The odds given by a simple combinatorial equation are 1 in 10^21 for a 10-million-year-old earth, and far smaller for a younger earth. [See pp. 376-8 for the details].
Number (2) is more interesting. Were the missing nuclides never created because the formation processes selected against them? Dalrymple proceeds through a detailed discussion of nucleosynthesis in sufficient detail to show that the missing nuclides are, in general, as likely as any other nuclides to be created by the known element-formation processes characteristic of stars and supernovae. In addition, all of the missing nuclides are easily made in nuclear reactors (p. 384). So, there is no known reason why they should not have formed, and many reasons why they should have.
Number (3) is the remaining option. The only feature common to all of these missing nuclides is their short half-lives (less than 80 million years).
This third hypothesis is testable.
First, by examining the nuclides and their half-lives in the table on p. 376, we can observe that their abundances are perfectly consistent with an Earth whose age is 4.5 billion years, but inconsistent with an age much younger. For example, an age of 1 billion years would be too young to explain the absence of Sm146 and the other four nuclides with half-lives between 10 and 80 million years.
Second, if these nuclides were ever around, their daughter isotopes should be present in amounts predicted by radioactive decay theories. And indeed there is some evidence: In the case of Iodine129 and the presence of 30x concentrations of its decay product Xe129, the most obvious explanation for all this extra Xe129 is that it came from Iodine129 that was originally present.
Questions…?
Is the decay constant actually a constant? [pp. 86-90 in AotE]. Conclusion is that yes, for all practical purposes (and in particular, for the purpose of measuring the age of the earth), decay rates are constant.
Bonus material: What about the RATE findings?
From Do the RATE findings negate mainstream science? (originally posted on talk.origins.org)…
Some young-earth creationists promote a research project funded in part by the Institute for Creation Research (ICR) called “Radioisotopes and the Age of the Earth”, or RATE. They describe the RATE findings as “astounding”, “successful beyond all expectation” and “history in the making”. RATE researchers claim to have uncovered powerful evidence confirming the Earth is young and demonstrating the old-earth model is not supported by empirical science. However, the methods and conclusions of this effort have been thoroughly debunked. For example…
Dinosaurs and mammals would have had to swim for months
The RATE conclusions hinge on their classification of the Paleozoic/Mesozoic strata as Flood rock. While the RATE team admits the exact boundaries of how the geologic column relates to the Flood are still under investigation, for the purposes of their study they claim that (from deepest to shallowest):
- Precambrian rock (dated 4.5 billion to 543 million years old) is pre-Flood deposits,
- Paleozoic rock (dated 543 to 248 million years old) is early Flood deposits,
- Mesozoic rock (dated 248 to 65 million years old) is mid to late Flood deposits, and
- Cenozoic rock (dated 65 million years old to present) is late and post Flood deposits.
However, the Precambrian layer contains only simple sea creatures, the Paleozoic contains insects and small primitive land creatures, the Mesozoic contains fossils of every dinosaur, and the Cenozoic contains the vast majority of mammal fossils.
Therefore, based on the RATE interpretation of the fossil record, all dinosaurs must have survived global floodwaters for over six months, and mammals for nearly a year. To put it another way, mammals must be able to swim longer than all dinosaurs, because nearly all mammals are sorted to the top, above nearly all dinosaurs– and both dinosaurs and mammals must have spent at least six months swimming! (It also seems odd that sea creatures would have immediately succumbed to being submerged in water … ???)
Heat from radioactive decay
The RATE team admits the geologic record unequivocally shows, at the very least, millions of years’ worth of radioactive decay, and they also acknowledge the energy that has to be associated with that much radioactivity.
They hence acknowledge this problem: If millions of years’ worth of radioactive decay occurred during the proposed interval of time (it would all have happened in the early weeks of the Flood, according to the RATE interpretation of the rock record), the heat from such a concentrated burst of radiation would have heated the entire Earth to many tens of thousands of degrees, and Earth’s surface would have been molten–vaporizing any water on the surface and ruling out the possibility of a global flood.
To deal with the enormous heat that would be generated by a tremendous burst of accelerated decay, the RATE team postulates “cosmological cooling”, an evidence-free concept involving general relativity, higher dimensions and a rapid expansion of space. They hypothesize that a temporary universal stretching of space occurred during the Flood--an expansion 20-fold times or greater--and that the heat generated by accelerated nuclear decay must have somehow been drawn into the fabric of space.
“A temporary universal stretching of space”…with no evidence to suggest this ever happened. I am reminded of Augustine’s observation regarding “outlandish statements about worldly things.”
Randy Isaac of The American Scientific Affiliation (ASA) provides a thoughtful review of the RATE work, concluding “…the expectation of a future solution to a major scientific impasse is being translated into conferences, books, and videos proclaiming the good news that the RATE project has demonstrated the scientific validity of a young earth…claims that [RATE’s] scientific data affirm a young earth do not meet the criterion of integrity in science. Any portrayal of the RATE project as confirming scientific support for a young earth contradicts the RATE project’s own admission of unresolved problems.”